Molar Mass of Nickel Iii Sulfate
The student then mixes the solution with excess AgNO3 solution causing AgCl to precipitate. The student collects the precipitate by filtration dries it and records the data shown below.

Solved Section Name 1 Calculate The Molar Mass Of Nickel Chegg Com
Approximately 40000 tonnes were produced in 2005.
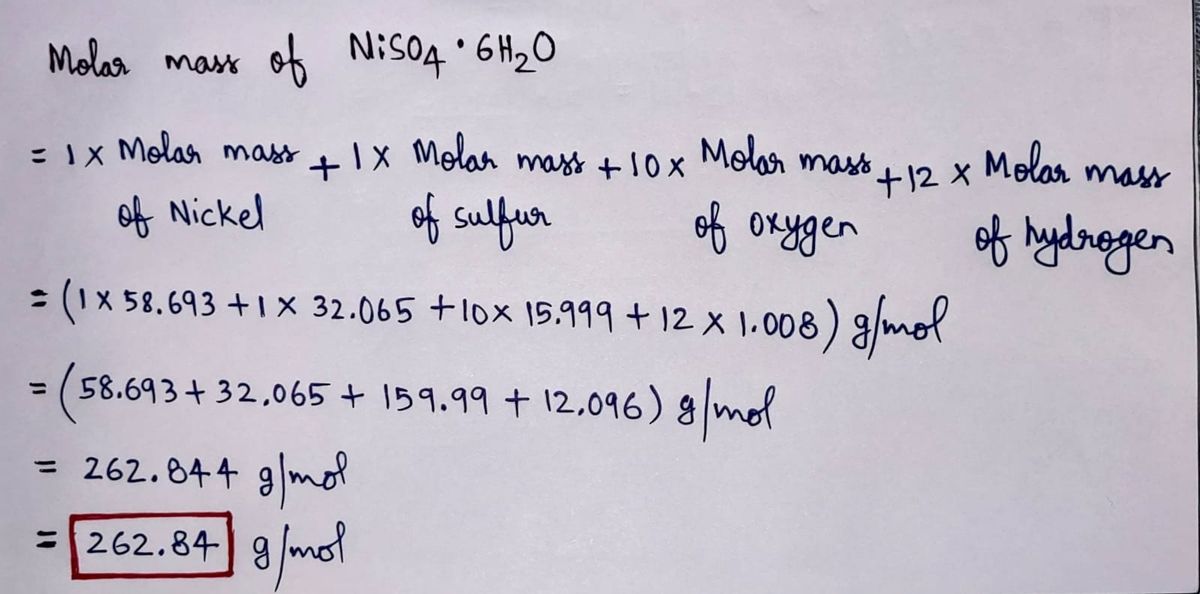
. Molar Mass of the compound is 14831 grams per mol. Chlorobenzene and bromobenzene form essentially ideal solutions in all proportions. Cd 3 AsO 4 2.
NickelII sulfate or just nickel sulfate usually refers to the inorganic compound with the formula NiSO 4 H 2 O 6This highly soluble blue green coloured salt is a common source of the Ni 2 ion for electroplating. In 20052006 nickel sulfate was the top allergen in patch tests 190. It is a black solid that is produced by treating nickelII salts with hydrogen sulfideMany nickel sulfides are known including the mineral millerite which also has the formula NiSAside from being useful ores nickel sulfides are the products of desulfurization reactions and are sometimes used as catalysts.
Nickel sulfide is an inorganic compound with the formula NiS. It is mainly used for electroplating of nickel. The molar mass of AgCl is 143 gmol Mass of unknown chloride MCl 074 g Mass of filter paper 080 g Mass of filter paper plus AgCl precipitate 223 g.
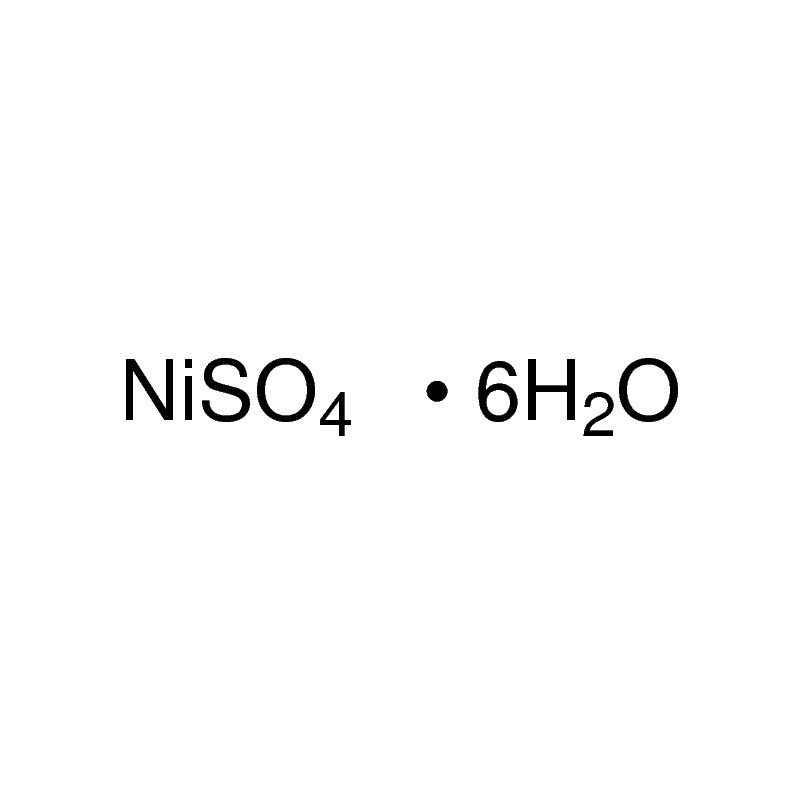
Nickel Ii Sulfate Hexahydrate 99 0 10101 97 0
Nickel 3 Sulfate 2 3 Ni2o12s3 Chemspider

No comments for "Molar Mass of Nickel Iii Sulfate"
Post a Comment